Describe the Arrangement of Ions in a Crystal Lattice
They conduct electricity because the charged particles are free to move. The simplest arrangement is one in which.

Crystal Lattice Structure Formation Expii
Describe the crystal structure of iron which crystallizes with two equivalent metal atoms in a cubic unit cell.

. This crystalline structure has a certain order creating a crystal lattice. Instead they have a regular repeating arrangement called an ionic lattice. A regular arrangement of ions The ions in a solid ionic compound are not randomly arranged.
Compute ionic radii using unit cell dimensions. The unit cell is the smallest three-dimensional portion of a complete space lattice that repeatedly in different directions produces the complete space lattice. The length relationship is the measured lengths of the sides of unit cells in a crystal.
These forces makeit difficult for one layer to move relative to another causing ionic compounds to be hard. Their arrangement varies depending on the ions sizes or the radius ratio the ratio of the radii of the positive to the negative ion. To visualize this imagine taking a large number of identical spheres such as.
Describe these conditions and explain why ionic compounds are not always used as conductors. This is why solid ionic compounds form crystals with regular shapes. Such as png jpg animated gifs.
The ions have a regular repeating arrangement called an ionic lattice. Let us begin our investigation of crystal lattice structure and unit cells with the most straightforward structure and the most basic unit cell. The crystal lattice is the arrangement of atoms in a crystal.
These atoms or groups of atoms are commonly referred to as points within a crystal lattice site. THE ARRANGEMENT OF IONS IN A CRYSTAL LATTICE comes in many forms. A regular arrangement of the constituent particles- atoms ions or molecules of a crystal in a three-dimensional space is called crystal lattice or space lattice.
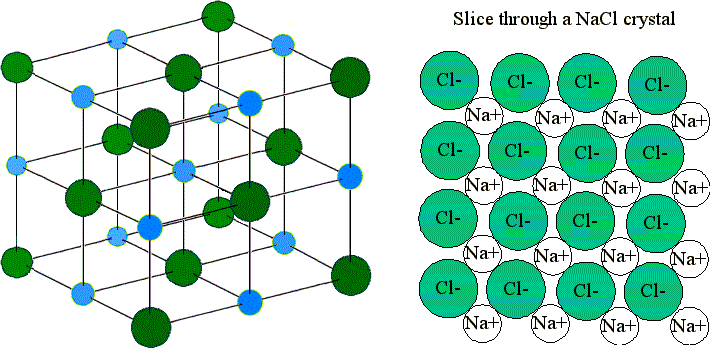
Each chloride ion is. The easiest way to picture such an array is to arrange one layer of spheres and then place successive layers over it. Each sodium ion is touched by 6 chloride ions.
Describe the arrangement of atoms and ions in crystalline structures. Describe the arrangement of ions in a crystal lattice. We Have got 15 images about Describe The Arrangement Of Ions In A Crystal Lattice images photos pictures backgrounds and more.
It can be simple cubic body centered cubic face centered cubic. The lattice is formed because the ions attract each other and form a regular pattern. Compute ionic radii using unit cell dimensions.
This arrangement is called a face-centered cubic FCC solid. The arrangement of sulfide ions is identical to the arrangement of chloride ions in sodium chloride. The crystal lattice is the symmetrical three-dimensional structural arrangements of atoms ions or molecules constituent particle inside a crystalline solid as points.
Most monatomic ions behave as charged spheres and their attraction for ions of opposite charge is the same in every direction. Ionic crystals consist of two or more different kinds of ions that usually have different sizes. Stability of ionic solids depends on lattice energy which is released in the form of heat when two ions are.
One-eighth of an atom at each of the eight corners 8 1 8 1 8 1 8 1 atom from the corners and one-half of an atom on each of the six faces 6 1 2 3 6 1 2 3 atoms from the faces. The ions have a regular repeating arrangement called an ionic lattice. A FCC unit cell contains four atoms.
In such page we additionally have number of images out there. It can be defined as the geometrical arrangement of the atoms ions or molecules of the crystalline solid as points in. This structure contains sulfide ions on the lattice points of an FCC lattice.
The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other. Heisenbergs uncertainty principle is used to describe the relationship between the momentum and position of an electron. A lattice is a term given to an ordered arrangement of points in a 3D shape creating a regular arrangement of atoms and ions.
Atoms molecules ions and other elementary particles. The structure of a crystal lattice consists of small unit cells. The packing of these ions into a crystal structure is more complex than the packing of metal atoms that are the same size.
The ions have a regular repeating arrangement called an ionic lattice. Describe the arrangement of atoms and ions in crystalline structures. Each positive ion is surrounded by negative ions which are surrounded by positive ions.
The structure of sodium chloride is in a lattice shape and is described as having a giant ionic structure. The concept of crystal packing assumes that the ions are hard spheres. A crystal lattice is the arrangement of these atoms or groups of atoms in a crystal.
Ions bound together by electrostatic attraction form ionic crystals. The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other. Inside the crystal lattice each sodium ion Na1 is surrounded by six chloride ions Cl1- and each chloride ion is surrounded by six sodium ions.
A simple cubic crystal lattice has ions equally spaced in 3D at 90 angles. The sodium and chloride ions in a crystal of sodium chloride form a crystal lattice. To visualize this imagine taking a large number of identical spheres such as.
In sold state ionic compounds. Describe the arrangement of ions in a crystal lattice. In an ionic solid the ions are packed together into a repeating array called a crystal lattice.
Describe the arrangement of ions in crystals in an ionic crystal even a slight shift of one row of ions relative to another causes a large buildup of repulsive forces. This is why solid ionic compounds form. Let us begin our investigation of crystal lattice structure and unit cells with the most straightforward structure and the most basic unit cell.
9 2 Ionic Bonding And Lattice Energy Chemistry Libretexts

Ionic Crystal Structures Chemistry For Non Majors

Gumball Coat Rack Multicolored In 2021 Gumball Sale Decoration Coat Rack
No comments for "Describe the Arrangement of Ions in a Crystal Lattice"
Post a Comment